
We are a recognized firm engaged in manufacturing an extremely efficient Reactor Vessel that is the central component of the reactor coolant system and which is sturdy steel constructed by the skilled professionals of our company. The Reactor Vessels designed and developed by us are widely used in pharmaceutical industries, chemical plants, etc.
Reaction vessels are at the heart of a chemical plant. They are where the chemical Changes and reactions take place. Powders or liquids may be poured into reaction vessels using large funnels, called hoppers, connected to the top of the vessel. These stainless steel liquid mixing tanks are highly efficient to use with their robust mixing structure for the homogeneous mixing of different chemicals and ingredients.
Types of Chemical Reactors:-

Batch reactors are used for most of the reactions carried out in a laboratory. The reactants are placed in a test tube, flask, or beaker. They are mixed together, often heated for the reaction to take place, and are then cooled. The products are poured out and, if necessary, purified. This procedure is also carried out in the industry, the key difference being one size of the reactor and the quantities of reactants. Following reaction, the reactor is cleaned ready for another batch of reactants to be added. Batch reactors are usually used when a company wants to produce a range of products involving different reactants and reactor conditions. They can then use the same equipment for these reactions. Examples of processes that use batch reactors include the manufacture of colorants and margarine.

An alternative to a batch process is to feed the reactants continuously into the reactor at one point, allow the reaction to take place, and withdraw the products at another point. There must be an equal flow rate of reactants and products. While continuous reactors are rarely used in the laboratory, a water softener can be regarded as an example of a continuous process. Hard water from the mains is passed through a tube containing an ion-exchange resin. The reaction occurs down the tube and soft water pours out at the exit. Continuous reactors are normally installed when large quantities of a chemical are being produced. It is important that the reactor can operate for several months without a shutdown. The residence time in the reactor is controlled by the feed rate of reactants to the reactor. For example, if a reactor has a volume of 20 m3 and the feed rate of reactants is 40 m3 h-1 the residence time is 20 m3 /40 m3 h-1 = 0.5 h. It is simple to control accurately the flow rate of reactants. The volume is fixed and therefore the residence time in the reactor is also well controlled. The product tends to be of a more consistent quality from a continuous reactor because the reaction parameters (e.g. residence time, temperature and pressure) are better controlled than in batch operations. They also produce less waste and require much lower storage of both raw materials and products resulting in a more efficient operation. Capital costs per tonne of product produced are consequently lower. The main disadvantage is their lack of flexibility as once the reactor has been built it is only in rare cases that it can be used to perform a different chemical reaction.
Types of continuous reactors:-
- Tubular Reactors
In a tubular reactor, fluids (gases and/or liquids) flow through it at high velocities. As the reactants flow, along a heated pipe, they are converted to products (Figure 4). At these high velocities, the products are unable to diffuse back and there is little or no back mixing. The conditions are referred to as plug flow. This reduces the occurrence of side reactions and increases the yield of the desired product. With a constant flow rate, the conditions at any one point remain constant with time and changes in the time of the reaction are measured in terms of the position along the length of the tube. The reaction rate is faster at the pipe inlet because the concentration of reactants is at its highest and the reaction rate reduces as the reactants flow through the pipe due to the decrease in the concentration of the reactant.
- Fixed Bed Reactors
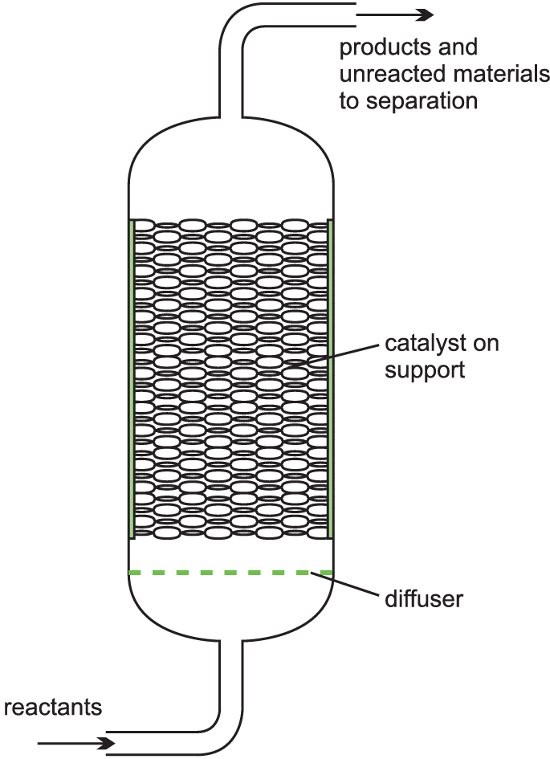
A heterogeneous catalyst is used frequently in industry where gases flow through a solid catalyst (which is often in the form of small pellets to increase the surface area). It is often described as a fixed bed of catalysts. Among the examples of their use are the manufacture of sulphuric acid (the Contact Process, with vanadium(V) oxide as a catalyst), the manufacture of nitric acid, and the manufacture of ammonia (the Haber Process, with iron as the catalyst). A further example of a fixed bed reactor is in catalytic reforming of naphtha to produce branched chain alkanes, cycloalkanes, and aromatic hydrocarbons using usually platinum or a platinum-rhenium alloy on an alumina support.
- Fluid Bed Reactors
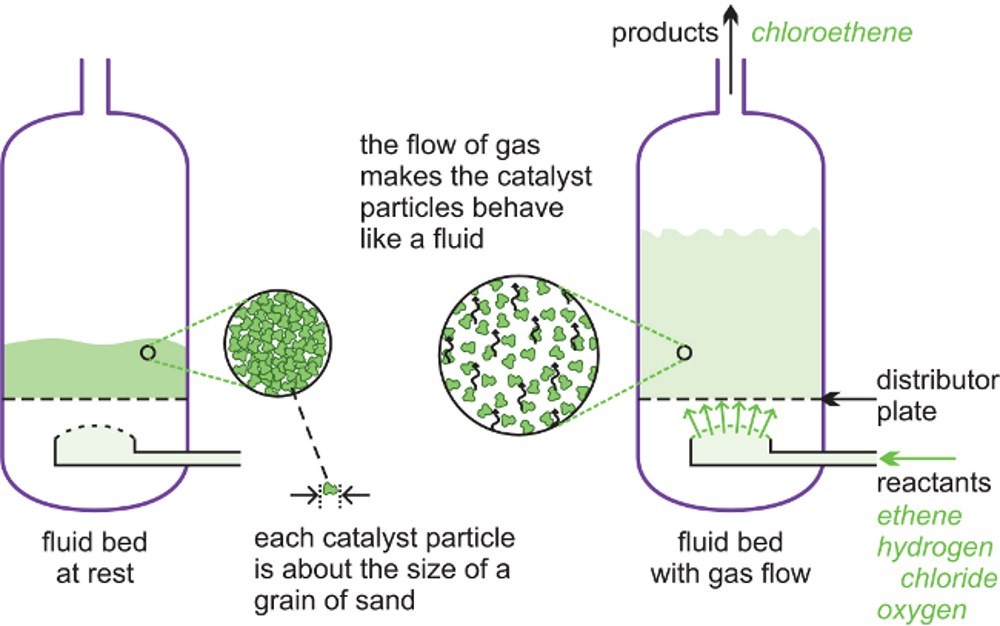
A fluid bed reactor is sometimes used whereby the catalyst particles, which are very fine, sit on a distributor plate. When the gaseous reactants pass through the distributor plate, the particles are carried with the gases forming a fluid. This ensures very good mixing of the reactants with the catalyst, with very high contact between the gaseous molecules and the catalyst and a good heat transfer. This results in a rapid reaction and a uniform mixture, reducing the variability of the process conditions. One example of the use of fluid bed reactors is in the ox chlorination of ethane to chloromethane (vinyl chloride), the feedstock for the polymer poly (chloromethane) (PVC). The catalyst is copper (II) chloride and potassium chloride is deposited on the surface of alumina. This support is so fine, it acts as a fluid when gases pass through it. Another example is the catalytic cracking of gas oil to produce alkenes (ethane and propene) and petrol with a high octane rating
- Continuous Stirred Tank Reactors

In a CSTR, one or more reactants, for example in solution or as a slurry, are introduced into a reactor equipped with an impeller (stirrer) and the products are removed continuously. The impeller stirs the reagents vigorously to ensure good mixing so that there is a uniform composition throughout. The composition at the outlet is the same as in the bulk in the reactor. These are exactly the opposite conditions to those in a tubular flow reactor where there is virtually no mixing of the reactants and the products.
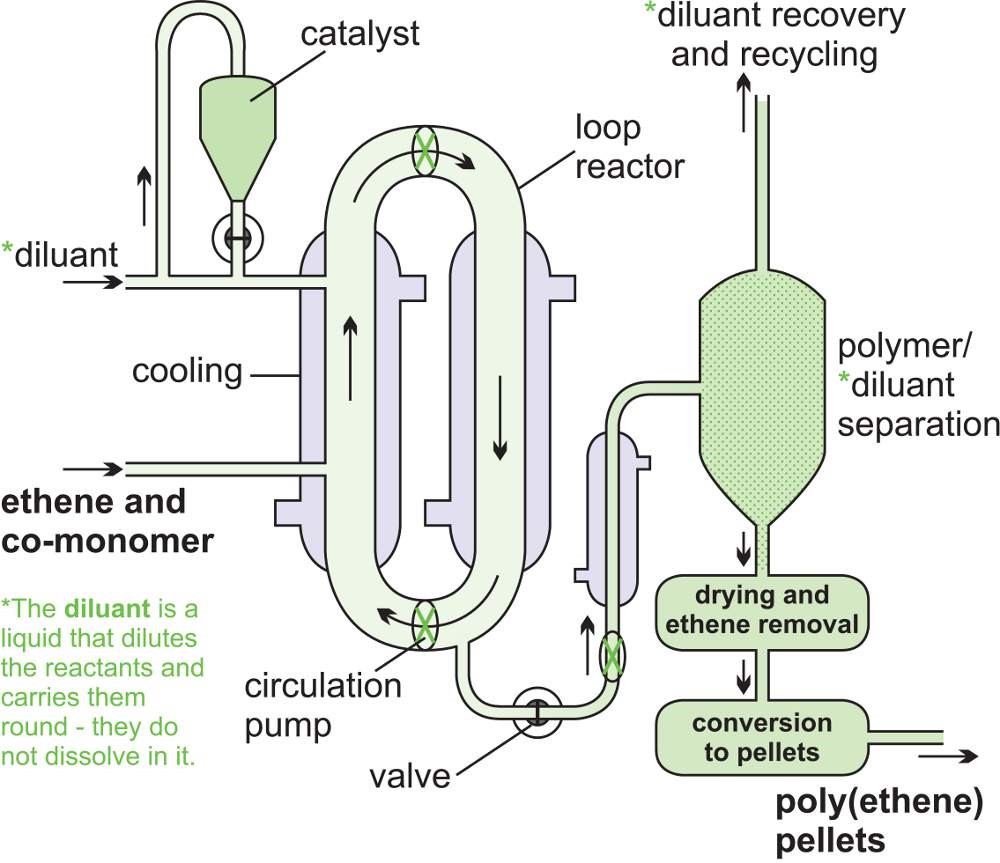
A steady state must be reached where the flow rate into the reactor equals the flow rate out, for otherwise the tank would empty or overflow. The residence time is calculated by dividing the volume of the tank by the average volumetric flow rate. For example, if the flow of reactants is 10 m3 h-1 and the tank volume is 1 m3, the time is 1/10 h, the residence time is 1/10 h, i.e. 6 minutes.
Get A Free Quote For Manufacturing & Fabricating
Contact us at the Industrial nearest to you or submit a business inquiry online.
Contact Us
